A Pipeline With the Potential to Impact Neurologic Diseases
The content on this page is intended for use by US healthcare professionals only.
The neurology pipeline includes investigational RNA-targeted medicines for severe and debilitating neurologic diseases that affect a wide range of people.5,6
Investigational RNA-targeted medicines (RTMs) are designed to target genetic sequences associated with neurologic diseases.1,7
Ionis’ Investigational Pipeline Includes Early- and Late-Stage Investigational Medicines for a Diverse Range of Rare and Prevalent Neurologic Conditions5,6,8,9,a
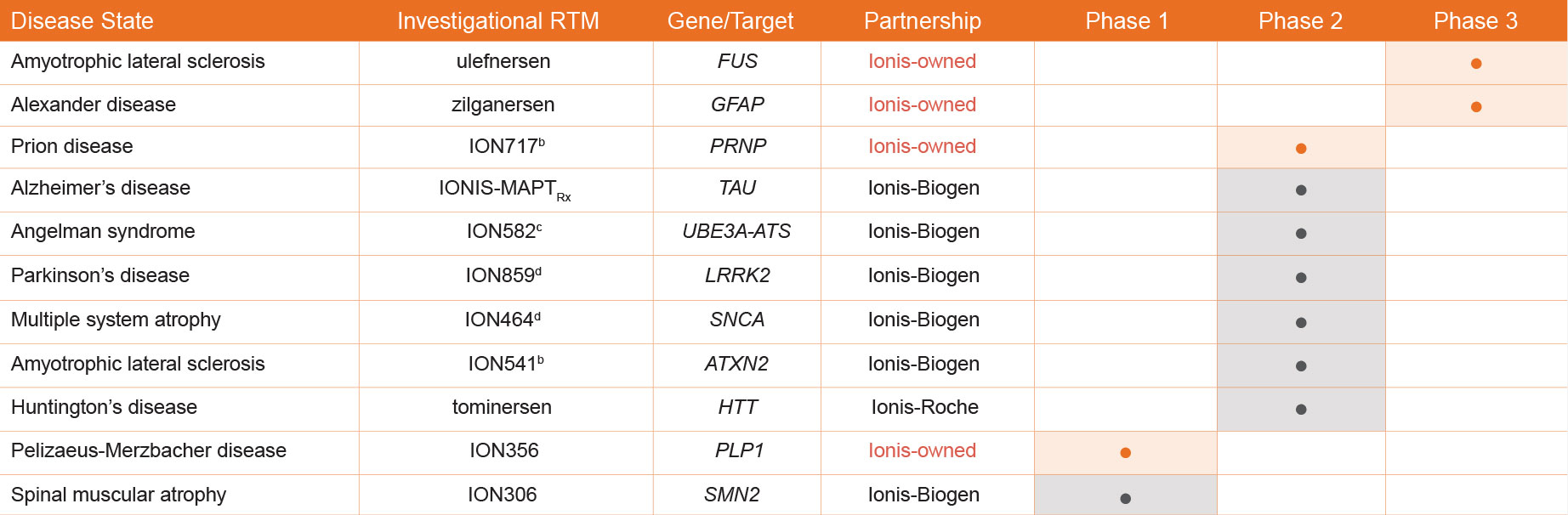
All agents listed above are investigational and have not been approved by the US Food and Drug Administration (FDA).1
aContent above subject to change pending updates to Ionis pipeline.
bThis investigational antisense medicine is in a Phase 1/2a study.2
cThe US Food and Drug Administration has granted both orphan drug designation and rare pediatric disease designation for its investigational drug ION582.3
dThis investigational antisense medicine is in a Phase 1/2 study. The primary purpose of the study is the evaluation of the medicine’s safety profile. It is listed here in Phase 2 because the medicine is being tested in patients and not healthy volunteers. This study may be categorized by partners or on regulatory sites, such as clinicaltrails.gov, as a Phase 1 study2
MECP2, methyl CpG binding protein-2.
Investigational RNA-Targeted Medicines in Clinical Trials
Ionis Neurology has several ongoing clinical trials applying investigational RNA-targeted medicines to a range of neurologic diseases.6
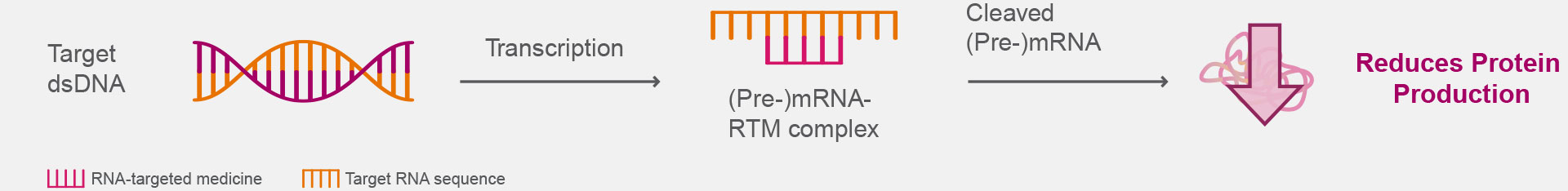
Current clinical trials are exploring investigational RNA-targeted medicines to target the genes associated with neurologic disease.4,6
Proposed RTM-Mediated Downregulation of Target Gene Expression4,10
If you are interested in learning more about the trial design, outcomes, and/or participating centers for one of the following diseases, please download the appropriate information sheet below.
If you are interested in more disease state education, please download the appropriate information sheet below:

