What’s Next: Innovation In Neurology
The content on this page is intended for use by US healthcare professionals only.
RNA-Targeted Regulation of Proteins
There are different classes of RNA-targeted medicines, including antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs).1
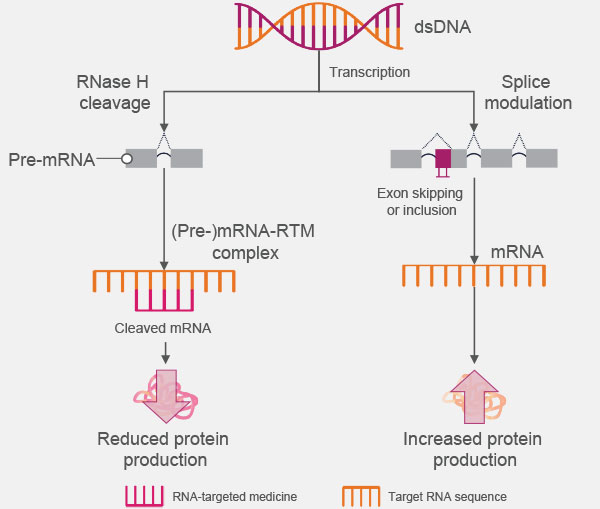
ASOs are single-strand oligonucleotides that bind directly to their mRNA target to upregulate or downregulate protein production by either1-4:
- Preventing protein translation through RNase H-mediated degradation of the heteroduplex (ie, RNA-targeted medicine–[pre-]mRNA duplex)
- Regulating gene expression via heteroduplex-induced alternative splicing, which includes exon skipping or inclusion
How Antisense Oligonucleotides Are Proposed to Work2,4
siRNAs are double-strand oligonucleotides that associate with the RNA-induced silencing complex (RISC) to bind the complementary target RNA, leading to altered protein production via mRNA cleavage or association-mediated repression.1,3,4
How siRNAs Are Proposed to Work5
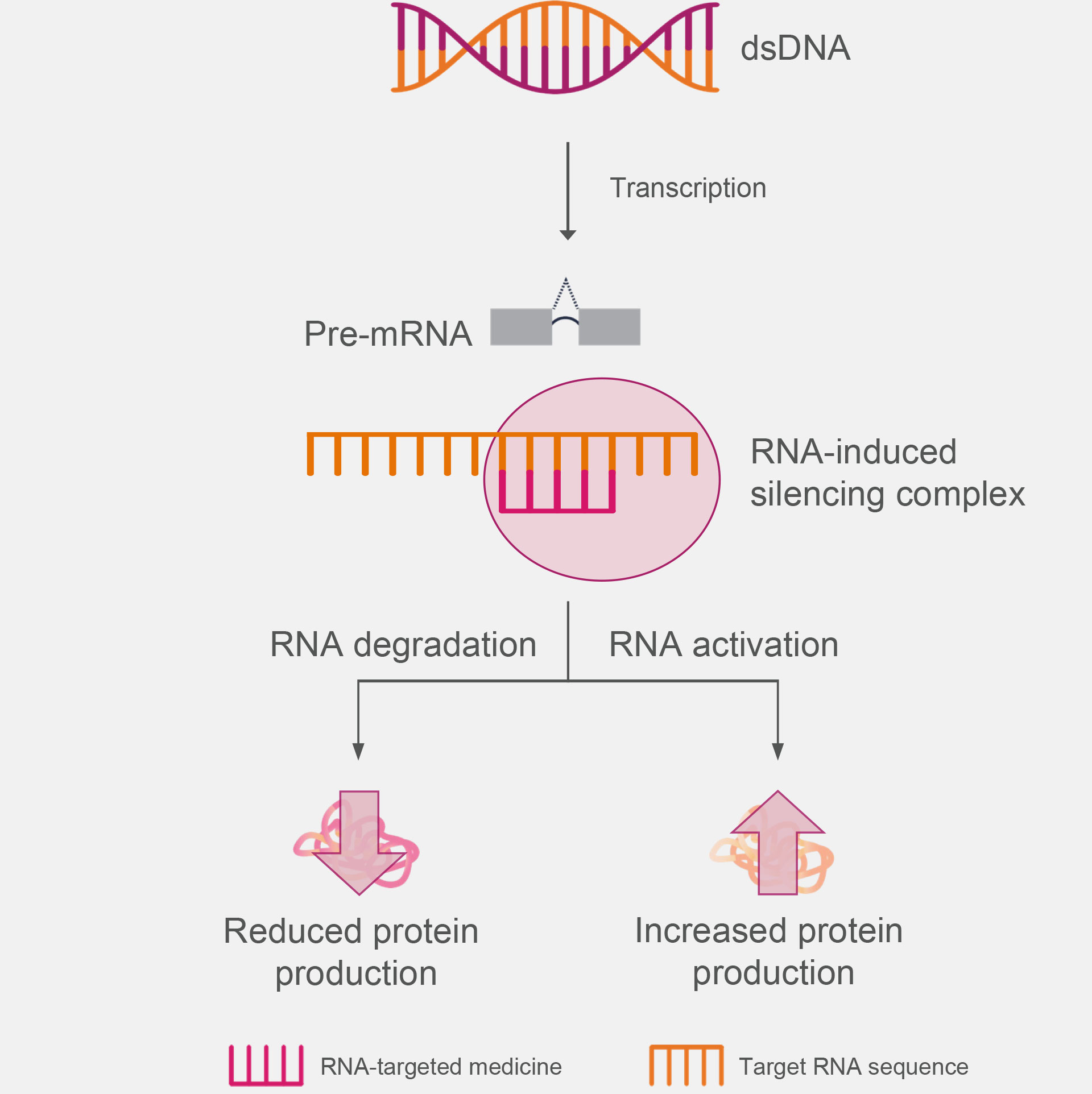
The molecular mechanisms through which RNA-targeted medicines modulate RNA function are dependent on the chemical modifications, the position of the modifications, and the location of the binding site on the target RNA.6
Creators of Trail-Blazing Innovation
Ionis created the chemistry behind RNA-targeted, FDA-approved, and investigational medicines and has spent decades advancing its platform.5,7-9
Ionis Has Decades of Experience Developing Therapies
for Hard-to-Treat Genetic Conditions
For decades, Ionis has worked to advance RNA-targeted technology to create medicines for rare and prevalent neurologic diseases including FDA-approved therapies such as nusinersen, eplontersen, and tofersen.5,10-21
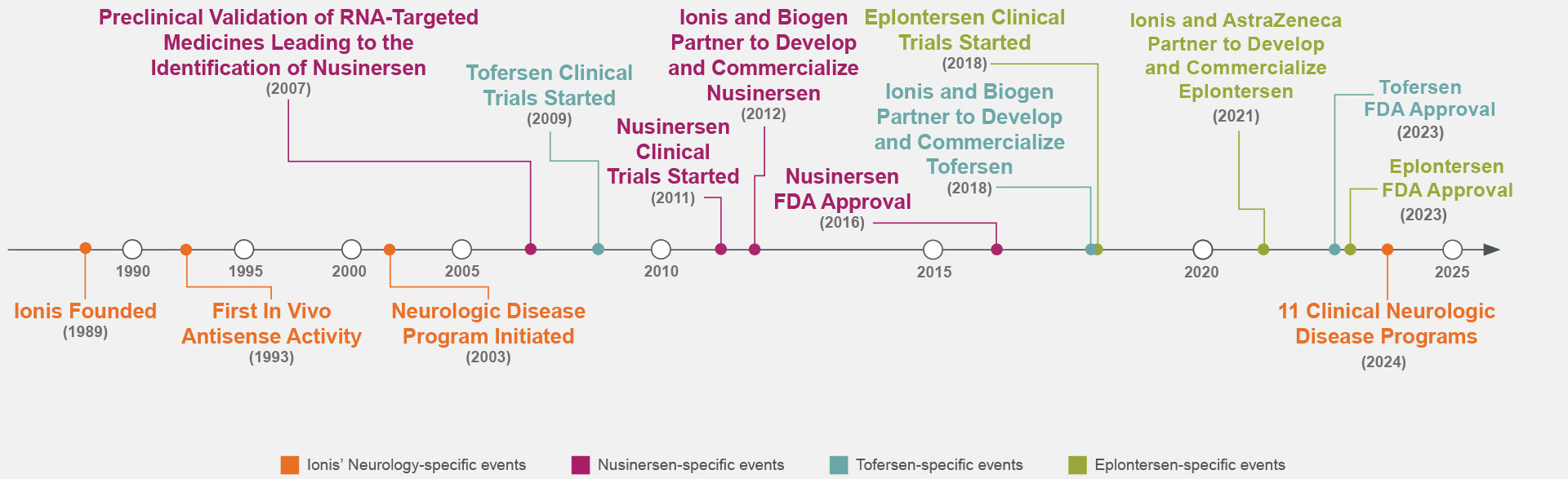
Ionis is a leader in developing transformational RNA-targeted medicines for neurologic diseases. Ionis advances include1,3,7,21-23:
- Developing the chemistry that is used as the basis for commercialized RNA-targeted medicines | Learn more
- Creating iterative screening and refining processes to identify RNA-targeted medicines for clinical settings | Learn more
- Innovating targeted tissue delivery and systemic delivery for RNA-targeted medicines
- Pioneering mechanisms to modulate gene expression
The success of nusinersen, eplontersen, and tofersen—all Ionis-originated, FDA-approved medicines—are examples of our ability to provide potentially life-changing options to patients with a neurologic disease.4,5,10,11,24
Taking RNA-Targeted Medicines Further
RNA-targeted medicines are designed to modulate mRNA in a highly specific manner. A comparative study of the efficacy, potency, and specificity of RNA-targeted medicines in a human cell culture assay found that as little as 2 base mismatches were enough to reduce RNA-targeted medicine activity.25
RNA-targeted medicine behavior can be modulated through chemical modifications, which has the potential to improve characteristics such as the potency, safety, and tolerability profiles.3,20,21
Optimization of RNA-targeted medicines at Ionis has led to20,21:
- Delivery to multiple tissue types
- Increased dose flexibility
- Increased safety and tolerability
- Decreased dose volume
- Increased potency
Click on the following figure to learn more about some of the pioneering biochemical discoveries behind the development of some of the first-in-class RNA-targeted medicines developed at Ionis.20
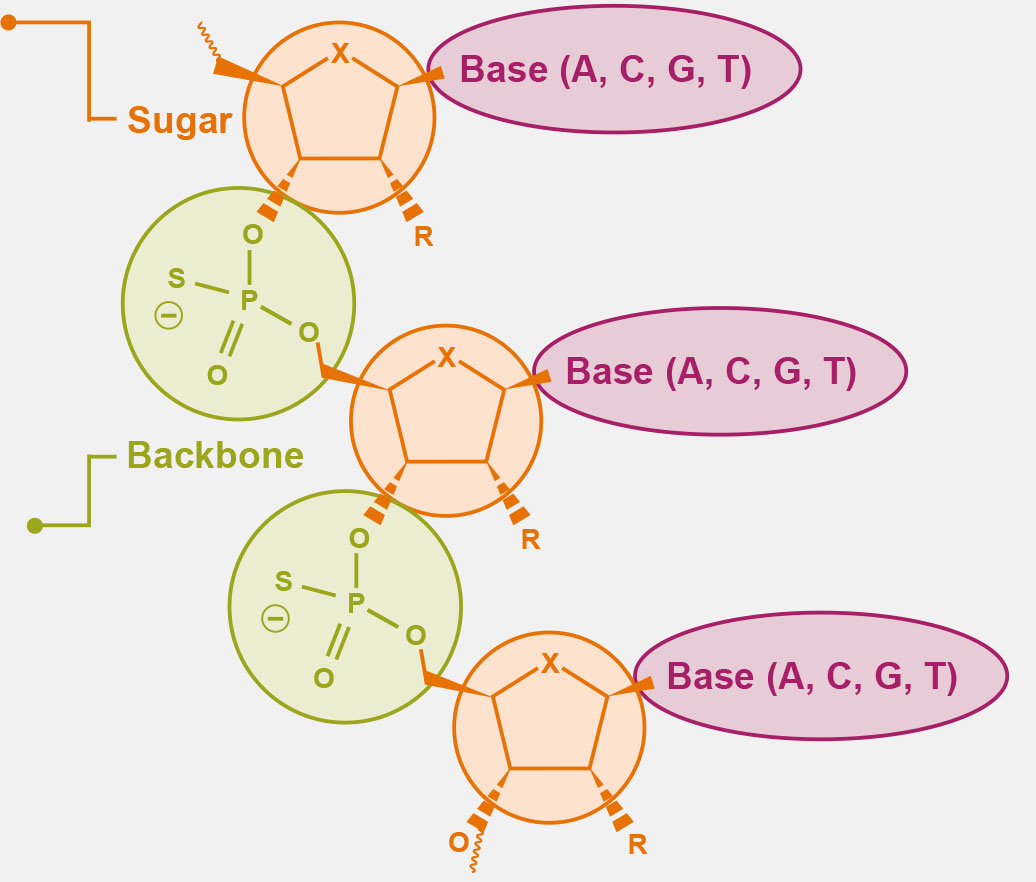
A, adenine; C, cytosine; G, guanine; T, thymine.
2’ Sugar Modifications and Conjugates
Modifications at the RNA-targeted medicine ribose position enhance the pharmacological profile and protein binding of investigational RNA-targeted medicines, leading to:
- 10-fold difference in protein binding
- Reduced RNA-targeted medicine toxicity
- Reduced degradation susceptibility
- Increased duration and effect
- Decreased inflammatory response
Backbone
Ionis pioneered the discovery and development of modifications to the oligonucleotide backbone of RNA-targeted medicines for:
- Increased stability
- Extended half-life
- Facilitated RNAse activity
- Improved tissue penetration
- Improved binding interactions