What’s Next: Innovation In Neurology
The content on this page is intended for use by US healthcare professionals only.
Delivering RNA-Targeted Medicines Deep Within the Nervous System
Preliminary research has demonstrated that investigational RNA-targeted medicines can penetrate tissues throughout the central nervous system (CNS) and are active across a wide range of cell types in the central nervous system.1-6 This offers the opportunity to develop RNA-targeted medicines for many types of neurologic diseases.1-4
RNA-targeted investigational medicines can be delivered to the spinal cord and deep regions within the brain, including the hippocampus, pons, and amygdala.1-4
Dose Response and Accumulation of Investigational RTMs in Mouse CNS Tissue1,a,b
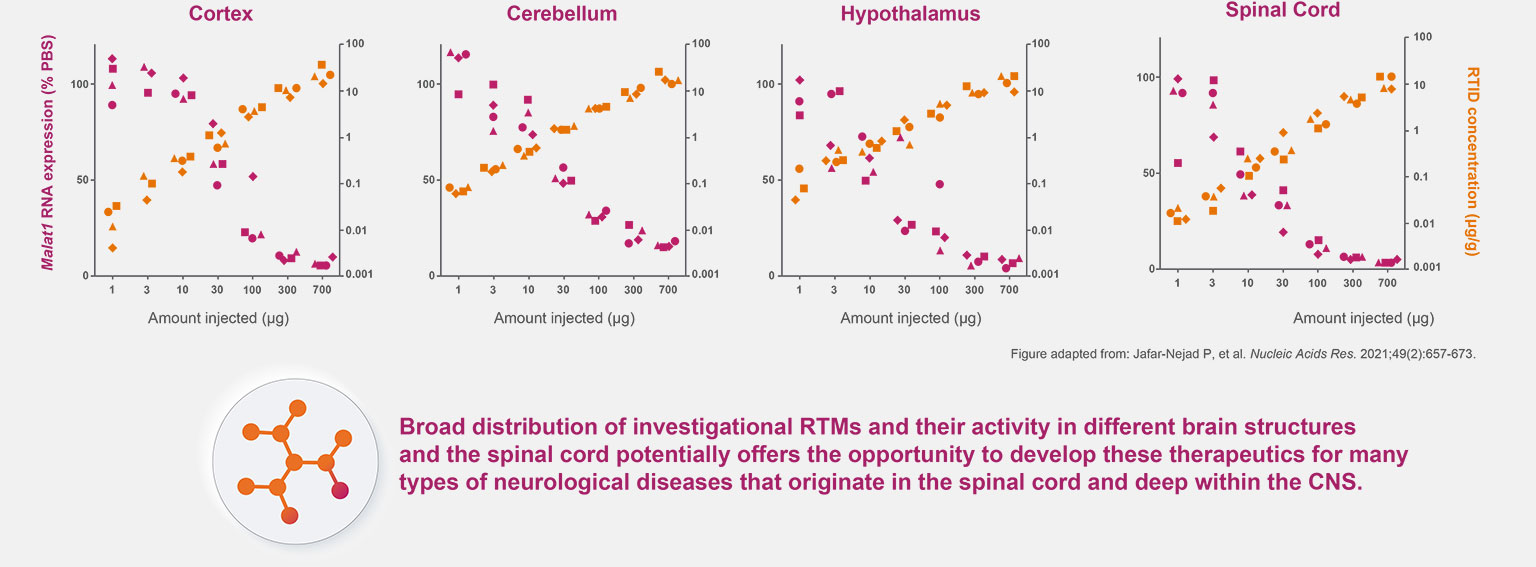
CNS, central nervous system; PBS, phosphate-buffered saline; RTID, RNA-targeted investigational drug; RTM, RNA-targeted medicine.
Research in animal models has shown investigational RNA-targeted medicines are active in neurons, microglia, astrocytes, and oligodendrocytes.1-4
In human patients, investigational RNA-targeted medicines have been shown to distribute within the CNS.7
Dose-Dependent Reduction of mRNA With Investigational RTMs
in All Four Major CNS Cell Types in Rodents1,a
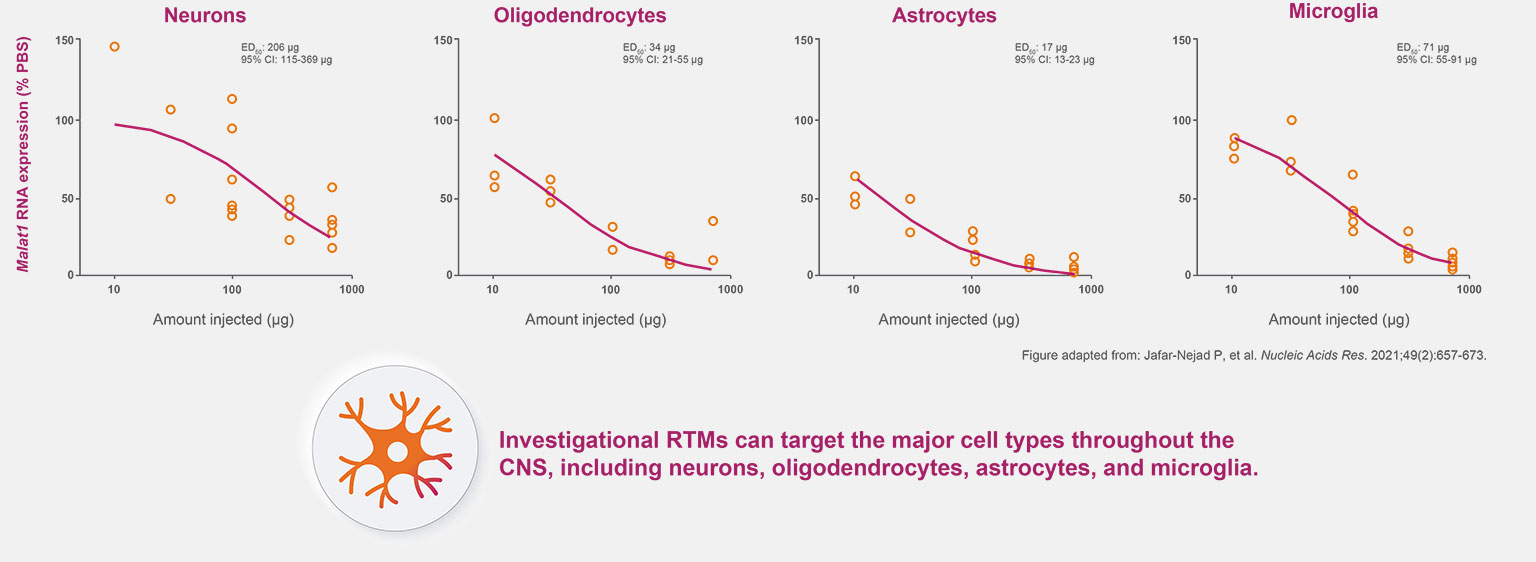
CNS, cnetral nervous system; ED50 effective dose; mRNA, messenger RNA; PBS, phosphate-buffered saline; RTM, RNA-targeted medicine.
To see how this technology functions in the CNS, please watch the video below:
With 4 FDA-approved neurologic medicines and more than 10 investigational RNA-targeted medicines in mid- or late-stage development, Ionis is potentially transforming the trajectory of serious neurologic diseases.8-11
Learn more about Ionis’ approach to screening investigational RNA-targeted medicines and its pipeline candidates.

