What’s Next: Innovation In Neurology
The content on this page is intended for use by US healthcare professionals only.
Transforming the Management of Neurologic Diseases
Discovering new approaches to manage neurologic diseases can be especially challenging. The current challenges with drug development leave significant unmet clinical needs among these patients.1
Ionis is committed to discovering, developing, and delivering potentially life-changing medicines for people living with severe, progressive, and fatal neurologic diseases.2
The Processes That Play a Role in Many Neurologic Diseases
Neurologic diseases can be caused by genetic variants from a range of proteins.3-6
Over 1700 distinct genetic variants have been identified as contributing to a range of conditions, including neurodegenerative, neuromuscular, and neurodevelopmental diseases.3-6
The types of genetic variants contributing to disease pathology can vary, but generally fall under 1 of 4 categories: point mutations, copy number variants, small insertions and deletions (InDels), and chromosomal rearrangements.7 These can cause disease through altering protein structure and/or production.7
Many Neurological Diseases Have a Genetic Component7
Genetic variants can vary, but generally fall under 1 of 4 categories:
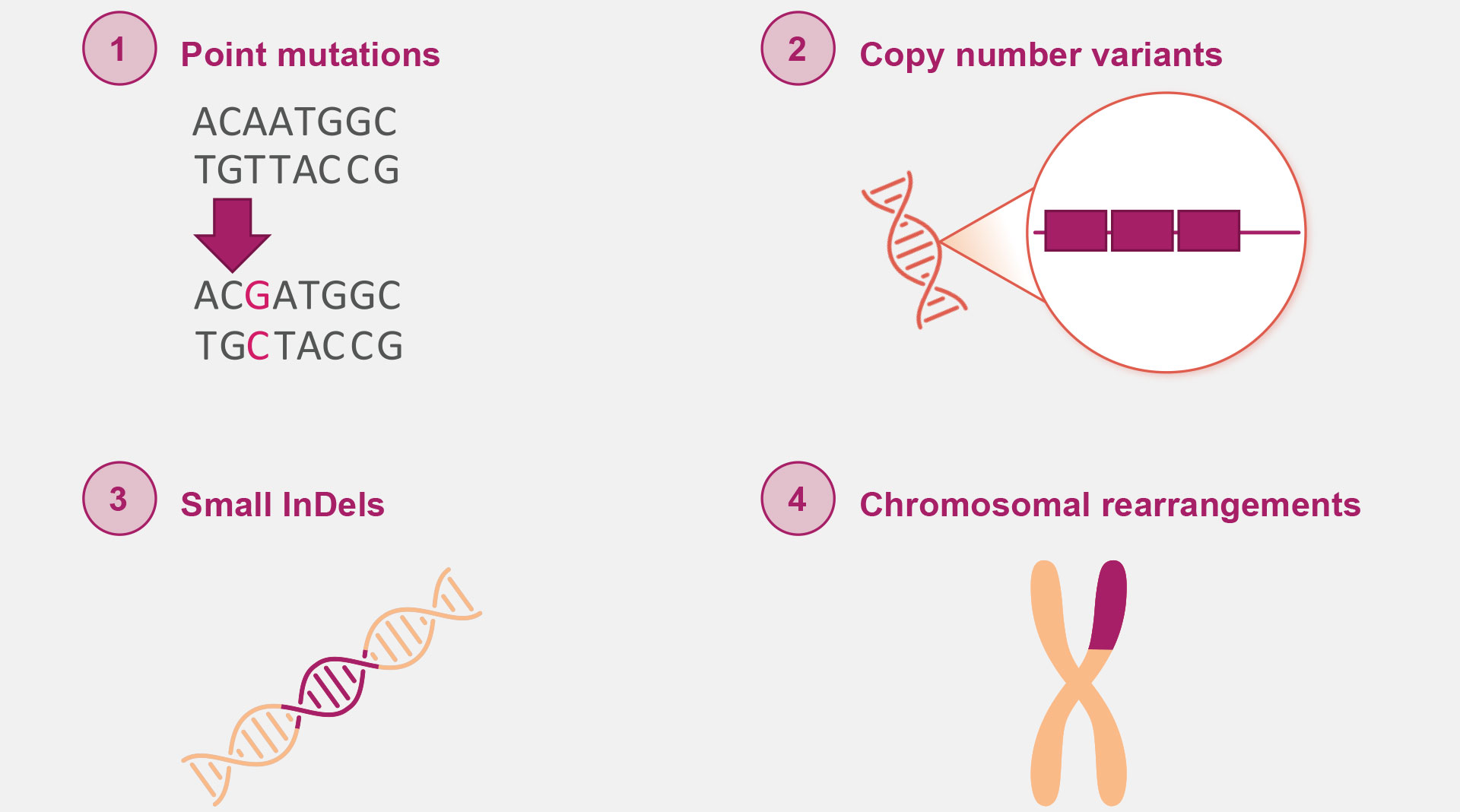
For example, point mutations or InDels in the glial fibrillary acidic protein (GFAP) gene can alter protein accumulation in Alexander disease while Angelman syndrome (AS) is primarily caused (70%) by chromosomal rearrangement causing loss of function of the maternally inherited ubiquitin protein ligase E3A (UBE3A) gene.8-10
Despite advancements in understanding the pathology of neurologic diseases, many are not readily targeted by traditional small molecules or antibody medicines.11
RNA has emerged as a unique target for developing therapeutics due to the broad applicability, translational utility, and efficiency of the drug discovery processes.12,13
Learn more about how RNA-targeted medicines work.
RNA-Targeted Medicines: Innovating What’s Possible
RNA-targeted medicines can target and modulate RNA in the cell, thereby altering protein production, and offer a therapeutic approach for neurological disease, including diseases that may not be amenable to treatment with small molecules or biologics.11,13-19
RNA-Targeted Medicines Alter Gene Expression Upstream of Protein Production Without Altering a Patient’s Genome14,16,17,19,20,21
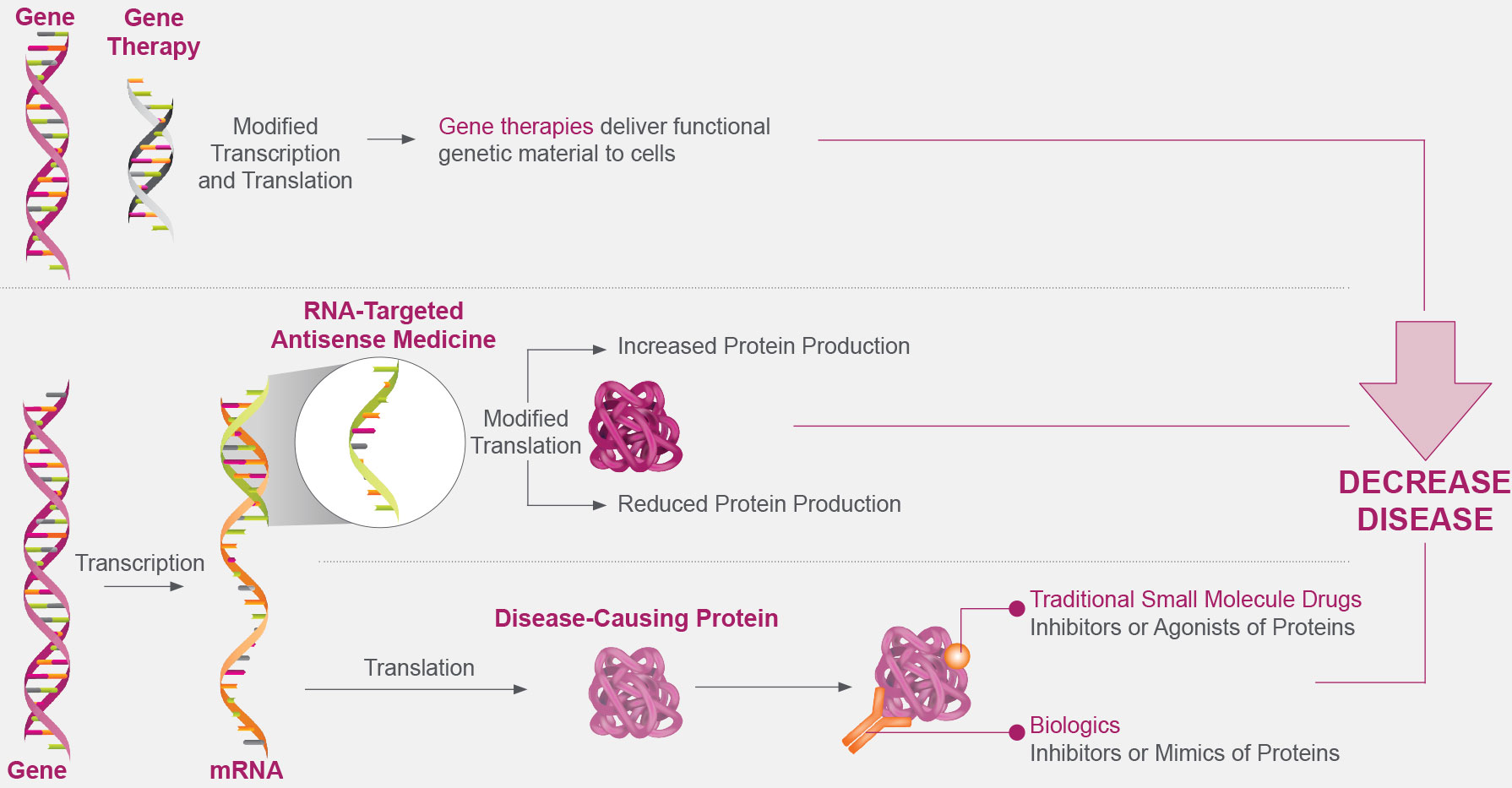
RNA-targeted medicines are designed to interact precisely with RNA through Watson-Crick base pairing.14,22 This allows RNA-targeted medicines to target a single disease-associated gene throughout the different tissues and cell types within the central nervous system (CNS).23 RNA-targeted medicines can modulate protein production primarily through the following 2 mechanisms11:
- mRNA degradation, either through RNase H-mediated or RNA-induced silencing complex (RISC)-mediated degradation
- Alternative splicing
The effects of RNA-targeted medicines on gene expression are rapid and reversible, and they do not modify a patient’s genome.11,12,22,24

